Hydrogen Peroxide is an unstable, colorless, or pale blue chemical compound which is generally used as an oxidizing and bleaching agent, a propellant, or antiseptic. It is about 1.5 times denser than water, very reactive, and flammable. It is miscible in water and forms hydrates, drifting from its pure substance due to the presence of hydrogen bonds in it. This naturally produced compound was first discovered in 1818, and now, industrially produced hydrogen peroxide is an indispensable element in various sectors - from old painting restoration to paper bleaching, from the healthcare industry to the garments industry, from weapons of bombardier beetles to satellites, and so on.
The biggest producer and exporter of hydrogen peroxide is Nederland; export value was around 103.5 million USD in 2020. Belgium, South Korea, Thailand, and Germany also join the list consecutively as the producers of hydrogen peroxide in significant amounts. On the other hand, Germany is at the top, followed by Japan, Chinese Taipei, and Russia, as the leading importers of this chemical. The total trade of hydrogen peroxide, whether or not solidified with urea - sums up the global trade worth 888 million USD, sharing 0.0053 per cent of the global trade. Bangladesh is also a producer as well as a regular importer of this chemical compound.
H2O2 Production and Consumption statistics in Bangladesh - Bangladesh exported 13.9 million USD and imported 656k USD of hydrogen peroxide in 2020.

H2O2 consumption sectors in Bangladesh -
The average production of H2O2 is around 190 Ton per day, whereas the actual demand is nearly 350 Ton per day. So, there is a considerable deficit between production and demand. That is why it needs to import hydrogen peroxide 50% (w/w) from India, Thailand, etc, to meet the demand.

Efficient and safe Production:
Hydrogen peroxide is industrially produced in the Anthraquinone process. But the process is very costly, especially for Bangladesh, and affects the ecology. Also, the production process is vulnerable and contamination happens. For hydrogen peroxide production in Bangladesh, alternative and eco-friendly processes can be implemented besides the Anthraquinone industrial process.
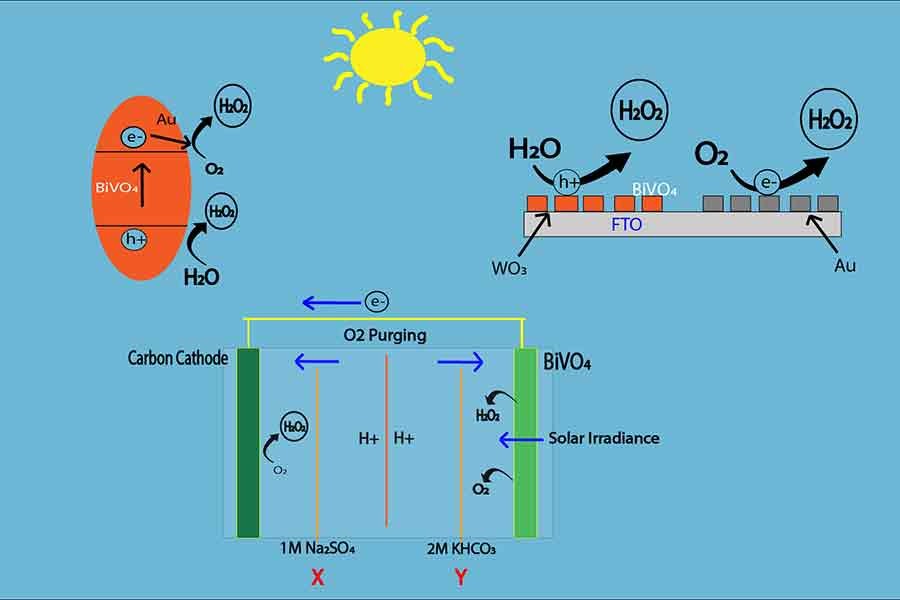
Photoelectric model:
It is eco-friendly and comparatively less expensive than Anthraquinone H2O2 production. In this process, Hydrogen peroxide is produced from water using energy from sunlight. In this model, BiVO4 or Bismuth Vanadate is used as a catalyst. It has a band gap of nearly 4.5 Volts, which makes it an effective photocatalyst. Carbon is used as the electrode in this photochemical reaction.
There are two chambers - Cathode (chamber X) and anode (chamber Y). The two chambers are detached by the membrane. The anode is open for sunlight but the cathode is away from the sunlight. When the Sun rays hit the photocatalyst BiVO4, the energy of light creates a bias of electrons to the cathode. As a result, to fill the gap of electrons in the electrode, water molecules in the anode chamber (chamber Y) release electrons, and H2O2 is created by oxidation reaction.
2H2O – 4e- → H2O2 + 2 [H]+
Then the electrons are conducted to the cathode chamber (chamber X). In the
cathode, the electrons also react with the water molecules and oxygen from the air
and creates H2O2.
H2O + O2 + 4e-→ H2O
Thus, hydrogen peroxide is produced from solar energy which is easier and less expensive than the conventional H2O2 production process, whereas the photo energy is gathered by BiVo4, which runs the whole production system.
Bacterial production model:
Hydrogen peroxide is formed in both prokaryotic and eukaryotic cells. But the accumulation of hydrogen peroxide is very hazardous for cells because it is toxic. So, most of the organisms of prokaryotic and eukaryotic cells neutralize H2O2 by enzymes. But these types of neutralizer enzymes are absent in some species such as S. pneumoniae. It has an incredible biological ability to resist hydrogen peroxide. Hydrogen Peroxide is a byproduct of the following biochemical reaction:
C3H4O3 (Pyruvate inorganic Acetyl) + (PO4)3- (Phosphate Phosphate) + O2 → C2H5O5P + CO2 + H2O2 [ catalyst - SPxB]
The pyruvate, inorganic phosphate, and oxygen react and creates acetyl phosphate, carbon dioxide, and hydrogen peroxide. SPxB enzyme is the catalyst of this reaction. S.pneumoniae is resistant to H2O2, so they do not need to neutralize hydrogen peroxide. This process is eco-friendly and biological and can be a good alternative to the production using Anthraquinone.
Efficient Transportation system for H2O2
Generally, a ‘tight-fill’ (closed-loop) loading operation is followed and can be transported into rail cars, tank trucks, or Iso-containers via 3” aluminum or stainless-steel loading arms or reinforced polyethylene chemical hoses. Tank cars can be unloaded via a self-priming pump. This is not ideal to unload rail cars by means of compressed gas because unwanted materials can cause auxiliary problems. Hydrogen peroxide should be shipped in 10,000-gallon insulated or non-insulated tank cars with safety valves and vents that meet the hazard specification.
While loading and unloading hydrogen peroxide, respective individuals must wear safety gear consisting of a hard hat with a brim, face shield, chemical-splash goggles, PVC or rubber gloves, and boots. Generally, centrifugal pumps, gear pumps, and single or double diaphragm metering pumps are used for H2O2 service. Only seal less pumps and pumps with single mechanical seals are glass-filled. PTFE on ceramic faces should be used. Double mechanical seals are not considered with hydrogen peroxide since they require barrier fluids which are often incompatible with hydrogen peroxide. In addition, they provide a space that can trap hydrogen peroxide.
Safe transportation also involves cleaning, pickling, passivating, and testing the metal surfaces which will come in contact with hydrogen peroxide. Moreover, the temperature, pH value, and presence of impurities are to be highly observed.
Efficient and safe Storage:
Hydrogen peroxide must be stored in a proper airtight container at 15 to 30 degrees centigrade. Strong sealed containers are responsible for H2O2 explosion as large pressure generates from the decomposition of H2O2 to water and oxygen. While carrying this chemical, interaction with heat and moisture as well as tightly closed containers, organic materials - acids, alkalis, metals - brass, copper, nickel, iron, bronze, zinc, synthetic rubbers, and polypropylene must be avoided. Aluminum 99.5%, stainless steel types 304, 304L, 316, and 316L, chemical glass, chemical ceramic, polytetrafluoroethylene, polyethylene, Viton®1, Kelf®2, Tygon®3, PVC, etc are recommended to use as primary and auxiliary materials to carry and store H2O2. Besides, to store H2O2 in a small amount - 250L two-door chemical safety cabinet, 1000L relocatable outdoor IBC storage unit can be used.
Conclusion
It requires rigorous investigation, field research, and data analysis to build up an integrated, safe, and efficient production, transportation, consumption, and storage model for hydrogen peroxide. And it becomes more important when it comes to a least developed country like Bangladesh. It is not about theories and laws, it is about the culture to maintain the standard practice with chemicals, both on an industrial or daily basis.
Abdullah Al Muhaimen
Notre Dame College, Dhaka
Md. Tauhidul Islam Tauhid
St. Joseph Higher Secondary School, Dhaka