Incepta Pharmaceuticals Limited, one of the country's leading pharma companies, has announced the launching of Pfizer's oral antiviral medication - Paxlovid - for treating Covid-19.
Incepta will market the drug under the brand name of Jupitavir, said a press release of the company on Wednesday.
The Directorate General of Drug Administration (DGDA) has approved emergency use of the drug to treat mild-to-moderate Covid-19 patients, including adults and children aged 12 years and above.
Earlier, the US Food and Drug Administration (FDA) granted emergency use authorisation to the drug on 22 December 2021 as the first approved oral therapy for Covid-19 treatment.
This novel antiviral pill showed almost 90 per cent efficacy in preventing hospitalisation and death of high-risk patients, according to the press release.
Citing recent lab data, it said the drug retains its effectiveness against fast spreading Omicron variant of the coronavirus.
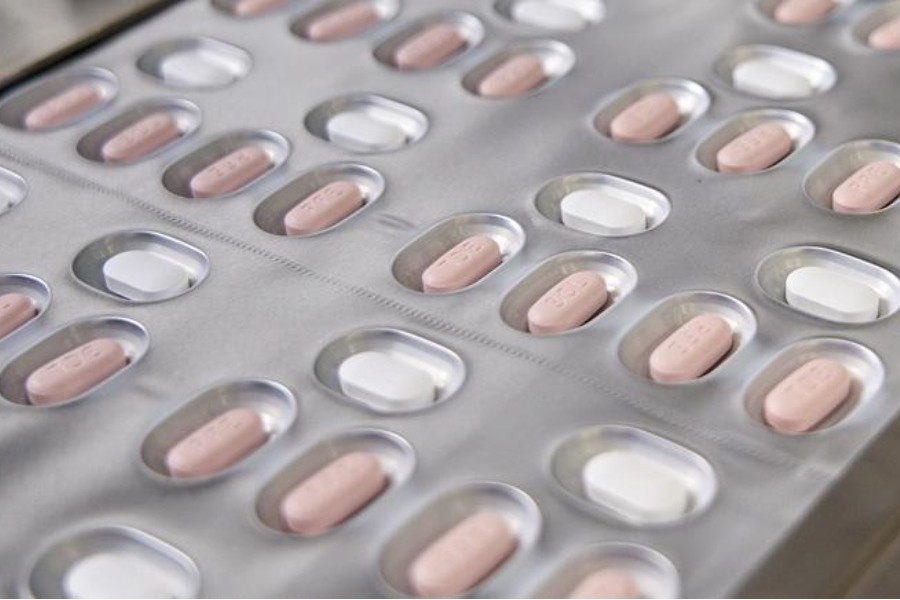
The drug is co-packaged with Nirmatrelvir and Ritonavir tablets.
Nirmatrelvir inhibits a SARS-CoV-2 enzyme to stop the virus from replicating, while Ritonavir slows down Nirmatrelvir's breakdown to help it remain in the body for a longer period at a higher concentration.
The treatment is given as two tablets of Nirmatrelvir and one tablet of Ritonavir together twice a day for five days.
Jupitavir is available by prescription only, and should be initiated as soon as possible after diagnosis of Covid-19 and within five days of symptom onset, the press release added.