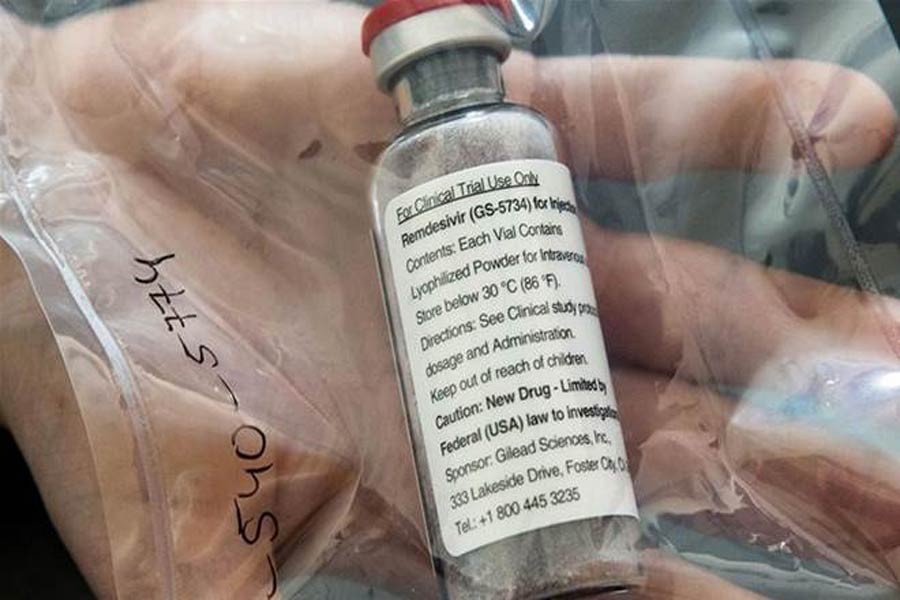
Antiviral drug Remdesivir is expected to be available this week to treat the COVID-19 patients, the CEO of the company manufacturing the medicine has said.
The Food and Drug Administration (FDA) of USA gave emergency use authorisation (EUA) for the use of investigational Remdesivir, produced by Gilead Sciences.
Earlier, some researches, including one led by Indian-American physician Aruna Subramanian, found that the drug helped recover some of the infected cases faster.
Gilead Sciences CEO Daniel O'Day said on CBS' "Face the Nation" on Sunday that its antiviral medication remdesivir will be sent to coronavirus patients (of USA) most in need early this week, reports Reuters.
”We intend to get that to patients in the early part of this next week,” O’Day said.
He said that his company has donated its entire available supply of the drug to USA, which can treat anywhere from 100,000 to 200,000 people of the country.
O’Day said the US administration will determine which cities are most vulnerable and where the patients need this medicine.
Earlier, US President Donald Trump said that his administration has been working with the teams at the FDA, National Institute of Health and Gilead for spearheading this public-private partnership to make this happen very quickly.
Remdesivir is authorised for the treatment of hospitalised patients with severe COVID-19 disease, it said, adding that the optimal duration of treatment is still being studied in ongoing clinical trials.
Under the EUA, both five-day and 10-day treatment durations are suggested based on the severity of disease.
The authorisation is temporary and does not take the place of the formal new drug application submission, review and approval process, Gilead noted.