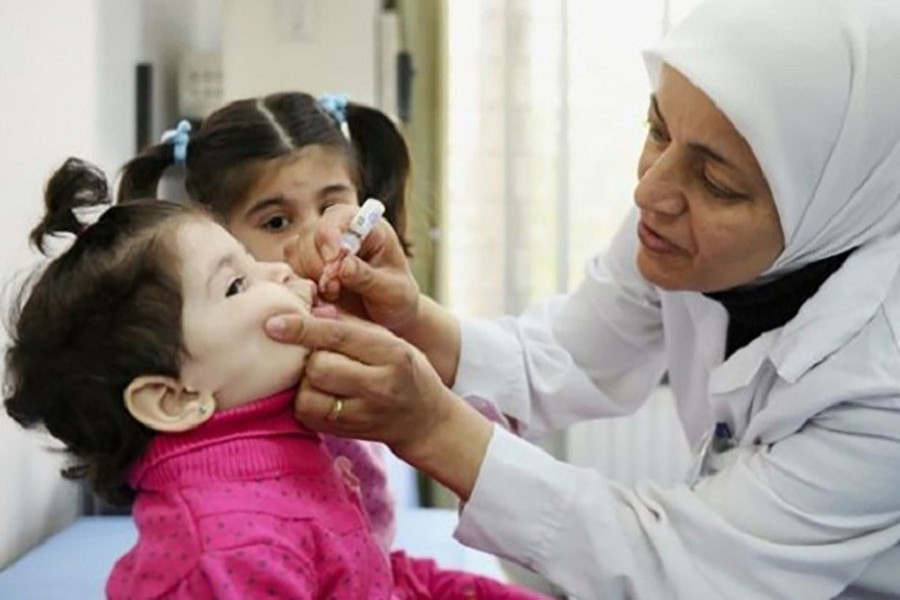
A new study by icddr,b scientists and partners published in The Lancet has shown for the first time that a novel oral polio vaccine developed for use in outbreak control is safe and leads to the production of productive antibodies in a population of newborn infants who are not previously exposed to any polio vaccine.
The randomised, double-blind, controlled, phase 2 trial was conducted in rural Bangladesh, at icddr,b’s Matlab Health Research Centre in Chandpur from 21 September 2020 to 16 August 2021.
The researchers identified women in their third trimester of pregnancy and invited them to enrol their infant in the study, according to a statement by the icddr,b on Sunday.
It evaluated the safety and tolerability, and immunogenicity after one and two doses of nOPV2 administration at 4 weeks apart to the infants. The researchers also looked at some secondary outcomes.
The vaccine was found to be immunogenic, resulting in 99% of infants having protective neutralising antibodies. Overall, 327 received two doses of vaccine or placebo and among these 325 were tested for immunogenicity per protocol. The vaccine was safe and adverse event rates were similar in the vaccine and placebo groups, it added.
Oral polio vaccines (OPVs) use live but weakened forms of the poliovirus to prime the immune system. On rare occasions, type 2 poliovirus used in traditional OPVs can mutate and reacquire neurovirulence — the ability to cause disease of the nervous system. Concerns about possible outbreaks of circulating vaccine-derived poliovirus type 2 (cVDPV2), led the Global Polio Eradication Initiative to withdraw type 2 poliovirus from OPVs and target only types 1 and 3.
The WHO recently authorised the use of a new OPV against type 2 poliovirus called nOPV2, which is less likely to revert to neurovirulence.
However, the vaccine had only previously been tested on individuals who had received at least one dose of the inactivated polio vaccine (IPV), which is less effective than OPVs at preventing transmission of the virus.
Thus the findings of this new study will equip public health experts and policymakers with the evidence to inform the use of nOPV2 in newborns with no previous exposure to other polio vaccines, who constitute the most vulnerable group for polio transmission.
Dr K Zaman, Senior Scientist in the Infectious Diseases Division at icddr,b, who led the study, says that “the novel oral polio vaccine is safe and immunogenic in the age group that most needs to be vaccinated to stop the chain of polio transmission in at-risk communities.”
“This information is particularly welcome given that over 450 million doses have already been distributed under the WHO Emergency Use Listing procedure, with no age restrictions for recipients,” he noted.
“Our study supports the continued use of the vaccine. However, there is still no data on how nOPV2 may interfere with Bivalent Oral Poliomyelitis Vaccine, the currently approved oral vaccine targeting types 1 and 3 poliovirus.”
Preventing the transmission of vaccine-derived poliovirus by vaccinating vulnerable vaccine-naïve newborns with nOPV2 is likely to be a key step toward poliovirus eradication.
The study was done in collaboration with the Bill and Melinda Gates Foundation, the US Centers for Disease Control and Prevention, the International Vaccine Institute in Seoul, and several other institutions, and funded by the Bill and Melinda Gates Foundation.