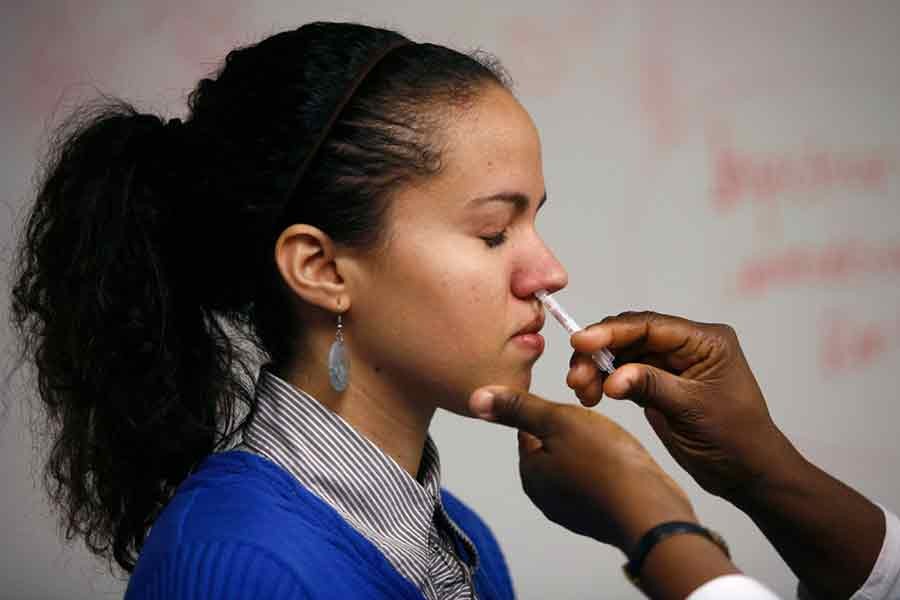Amid an unexpected surge in Covid-19 cases in some countries, the government of India has approved Bharat Biotech's first intranasal vaccine iNCOVACC as a booster dose for all adults.
Indian news outlets say Bharat Biotech's iNCOVACC will be available as a Covid-19 heterologous booster dose with two drops in a dose.
The country included the nasal vaccine in its Covid-19 vaccination programme on Friday. It will be available first in private hospitals.
In September, the nasal vaccine got emergency use authorisation from the Drugs Controller General of India (DCGI). The World Health Organisation (WHO) lauded the vaccine and said that it could help to bring the pandemic under control.
This vaccine is expected to be administered to those above 18 years of age. Details of the pricing and availability will soon be updated in the portal of the Indian government.
Bharat Biotech says it is BBV154, a novel adenovirus vectored, intranasal vaccine for Covid-19. It stimulates a broad immune response, neutralising IgG, mucosal IgA, and T-cell responses.