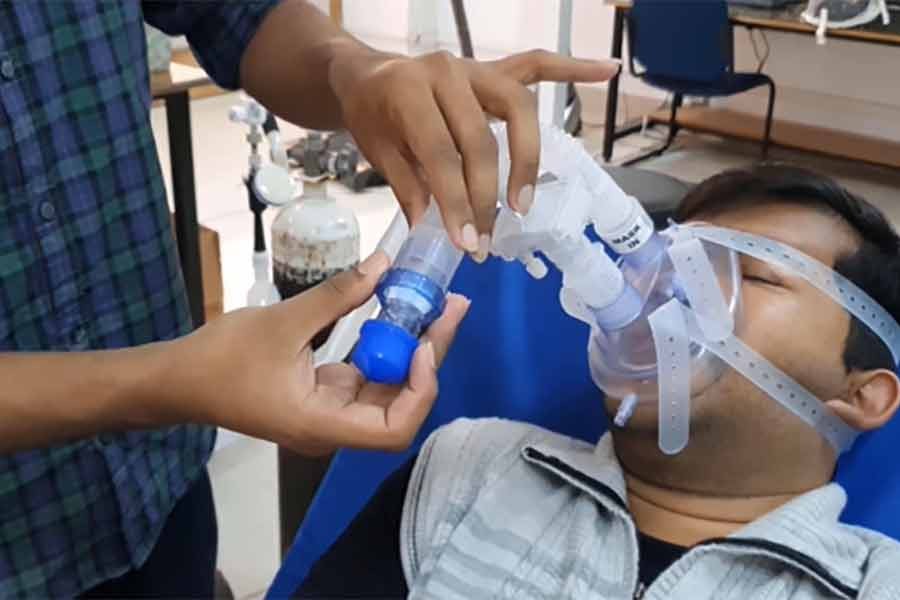
At a time when Covid-19 critical patients requiring high flow nasal oxygen (HFNO) treatment at hospitals are dying because they cannot be provided with the same, a Supreme Court lawyer brought the wrangling over manufacture of OxyJet to the High Court's notice. Devised by the Bangladesh University of Engineering and Technology (BUET), this low-cost medical implement has already successfully passed two-stage clinical trials and the third-stage assessment is going on. Its developer, the Department of Biomedical Engineering of the BUET asserts that the simple and portable device can be an alternative to the high-flow nasal cannula costing between Tk 200,000 and 500,000. A non-invasive continued positive air pressure (CPAP) ventilator, it can provide up to 60 litre 100 percent oxygen a minute depending on a patient's condition. It is easy to handle and does not require electricity for oxygen supply. Clearly, the positives are too many to be ignored.
Now, the lawyer complains that the Directorate General of the Drug Administration (DGDA) is unwilling to grant permission for its commercial production. On submission of his complaint, a virtual HC bench has directed the lawyer to write an application to the prime minister, observing that she is 'innovative and proactive' and might take necessary steps in this regard. Realising the urgency of the matter, the HC bench may have issued this unusual directive. In fact, the DGDA has a point here. The BUET is not an industry or company. It cannot receive a licence for commercial production of instrument --- medical or otherwise. Moreover, there arises the question of capacity for commercial production on the part of the BUET. Usually what happens is that universities receive fund for research, invention and development of many things for saving life, making living easier and comfortable as well as helping the humanity in distress. The collaboration between the OxfordUniversity and pharmaceutical company AstraZeneca for vaccine development may be a good example.
In the case of development of OxyJet, the BUET received fund from three organizations and one of them is the Innovation and Entrepreneur Development Academy Establishment under the ICT Division of the government. Why no company came forward with fund or is still not interested in commercial production of this highly prospective ventilator is really intriguing. The DGDA cannot dismiss the invention off-hand. In this case, the observation of the HC bench is illuminating. It remarks that such government organisations are more interested in importing costly instruments. Indeed, local inventions are given a cold shoulder. A Covid testing kit produced locally was not approved. Then a vaccine developed by a local company did not receive the attention it deserved. Even robots fabricated by school students for helping treatment of Covid patients and a ventilator devised by another student have not received enough patronage.
The bottom line is that talents are not nurtured, inventions are not encouraged. If the BUET-developed OxyJet have so many advantages and it still does not receive urgent approval for commercial manufacture, it will be a collective failure. So far its prospect looks bright --- even in terms of export. Currently, it costs about Tk 25,000 but once it is commercially produced the cost will definitely come down. As it does not require electricity, it will be highly useful for patients in outlying areas where the virus is on the rampage. It is believed there will be intercession from the highest level in the matter for saving more lives.